Exploring CAR T-Cell Therapy: A Promising Breakthrough for treatment of MS and other Autoimmune conditions

Is CAR T-Cell Therapy the Future of MS Treatment ?
CAR T-Cell Therapy may change lives for MS patients ?
This innovative approach, originally successful in cancer treatment, is now showing promise in managing Auto immune conditions by reprogramming immune cells to target harmful immune responses.
Could this be the breakthrough we’ve been waiting for ?
In the ever-evolving landscape of medical science, few innovations have sparked as much hope—and debate—as Hematopoietic Stem Cell Transplantation (HSCT). What was once a treatment reserved for certain blood cancers has now emerged as a potential game-changer for those living with some of the most stubborn autoimmune diseases : Multiple Sclerosis (MS), Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), Myasthenia Gravis, and Scleroderma, to name a few.
What CAR T-cell therapy is and how it works ?
CAR-T cell therapy – is the breakthrough that got American scientist James P Allison and Japanese physician Tasuku Honjo the 2018 Nobel Prize in Medicine after more than two decades of work. CAR-T cell therapy is a type of immunotherapy that uses a patient’s own T-cells, a type of white blood cell, to fight cancer.
The process involves extracting T-cells from the patient’s blood, genetically engineering them in a lab to express a chimeric antigen receptor (CAR) that targets specific proteins on cancer cells, and then infusing these modified cells back into the patient. It has been a promising option for several types of cancers and is FDA approved as a therapy for leukemia, lymphoma, and multiple myeloma.
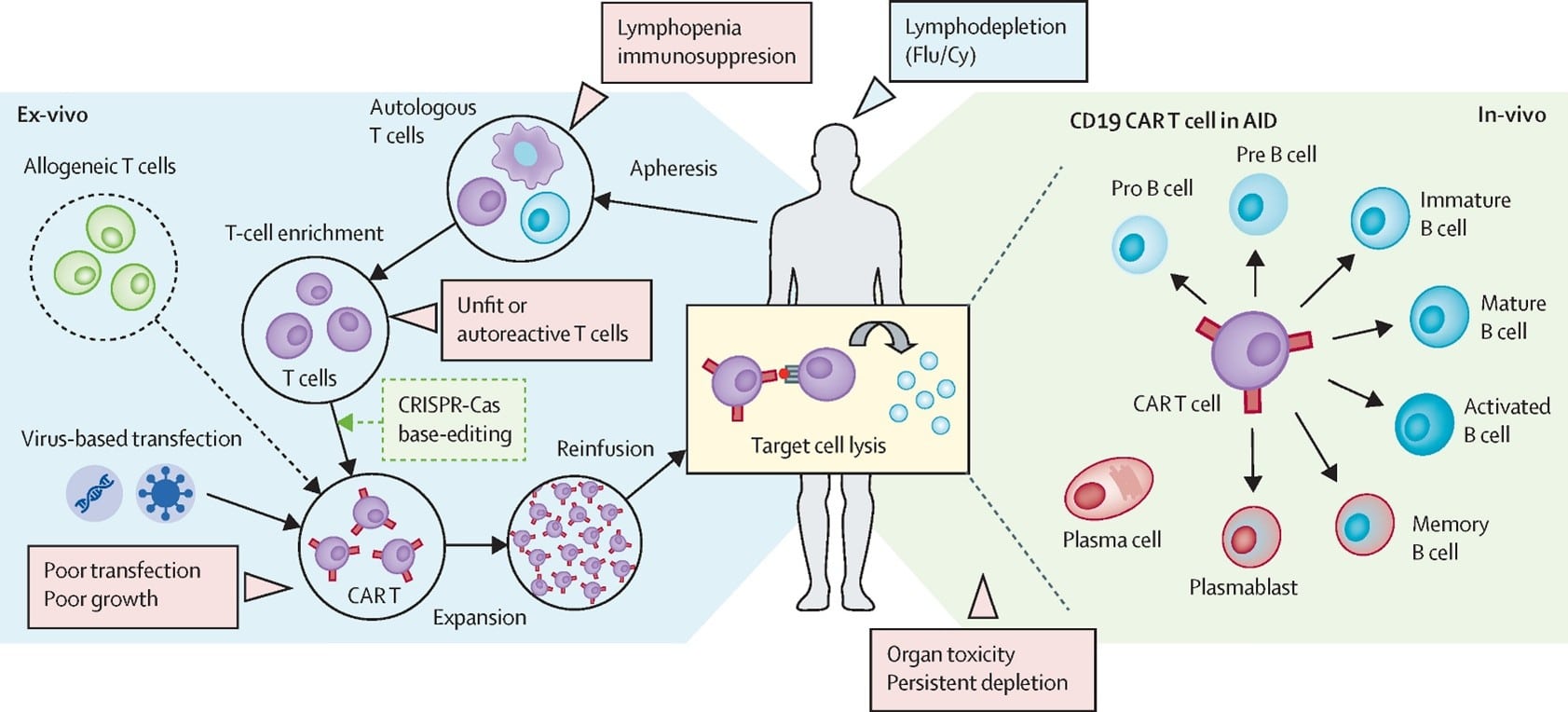
How could CAR T-cell therapy revolutionize MS treatment compared to existing options ?
Recent advancements in CAR-T cell therapy for autoimmune conditions through clinical trials,, particularly Lupus, have shown promising early results, with clinical trials expanding to explore its potential as a transformative treatment. 5 patients -four women and one man, aged 18 to 24—received transfusions of CAR T-cell therapy to treat severe lupus, an autoimmune disease that can cause life-threatening damage to the heart, lungs, brain and kidneys. This new study was led by rheumatologist Georg Schett of the University of Erlangen-Nuremberg in Germany.
The treatment sent the disease into remission for all 5 patients, allowing them to go off lupus medication for up to 17 months. Scientists and doctor hail the success as it raises hopes for tackling other autoimmune conditions such a rheumatoid arthritis and multiple sclerosis. However, further larger studies will be needed, but surely good news for these 5 people and hope for progress! The first patient to receive this treatment has been symptom-free for almost four years.
More than 40 people with lupus have now undergone new CAR-T cell therapy, and most have gone into drug-free remission. It is too early to declare any of these patients cured for life, but that now seems within the realm of possibility.
Insights from recent CAR T-cell therapy clinical trials for Autoimmune conditions and their results
Many New clinical trial with RRMS or progressive forms of MS are presently going on worldwide.
CAR-T-cell therapy was originally developed as a way to kill malignant cells in blood cancer,” Zhang continues. But now doctors have hope that it could offer a bigger breakthrough for autoimmune diseases beyond just lupus, including multiple sclerosis, myositis, and myasthenia gravis. “The success of CAR-T has inspired researchers to borrow other—cheaper and simpler—strategies from cancer therapy to kill immune cells gone awry.
CD19-Targeted CAR T-Cell Therapy in MS:
Feasibility and Safety: A small study published in Med (2024) reported the first use of fully human CD19-targeted CAR T-cell therapy (KYV-101) in two patients with progressive MS. The therapy demonstrated an acceptable safety profile, with no instances of immune effector cell-associated neurotoxicity syndrome (ICANS), despite CAR T cells being detected in the cerebrospinal fluid (CSF). This suggests the therapy can penetrate the central nervous system (CNS), a critical factor for MS treatment.
Efficacy: In one patient, intrathecal antibody production in the CSF decreased significantly and remained low through day 64 post-infusion, indicating a potential reduction in CNS inflammation. While clinical outcomes were not fully detailed, the presence and expansion of CAR T cells in the CSF without neurotoxicity suggest a therapeutic effect on B-cell-driven pathology.
Case Series Across Autoimmune Diseases: A pivotal study in the New England Journal of Medicine (2024) evaluated CD19 CAR T-cell therapy in 15 patients with severe autoimmune diseases, including systemic lupus erythematosus (SLE), idiopathic inflammatory myositis, and systemic sclerosis, but not MS. All eight SLE patients achieved remission per DORIS criteria, all three myositis patients showed major clinical responses (ACR-EULAR criteria), and all four systemic sclerosis patients had reduced disease activity (EUSTAR index).
Immunosuppressive therapy was discontinued in all patients, and B-cell depletion lasted an average of 112 days. Safety concerns were minimal, with most adverse events being grade 1 cytokine release syndrome (CRS). This study provides a rationale for controlled trials in MS, given shared B-cell-driven mechanisms.
Systemic Sclerosis: A case series in The Lancet Rheumatology (2024) detailed CD19 CAR T-cell therapy in patients with diffuse cutaneous systemic sclerosis. No progression of organ manifestations was observed over a median follow-up of 487 days, with improvements in the ACR-CRISS index and depletion of antinuclear antibodies. These findings highlight the potential for CAR T to reset immune homeostasis, which could be relevant for MS.
Benefits, Challenges, risks – How does the future of this cutting-edge CAR T cell therapy look like for Autoimmune conditions like MS, CIDP etc. ?
CAR-T or Chimeric Antigen Receptor T-cells. With this approach, T-cells from the patient are removed and reprogrammed in the lab to express a chimeric receptor. They are then given back to the patient by IV.. “Because each patient’s own cells are reengineered, it cannot be easily scaled up. Still, “even if CAR-T itself is never widely adopted for autoimmune diseases, it has opened the door to new ideas that could one day revolutionize their treatment.”
In the next few years could bring an inflection point in treating some of the most frustrating and intractable diseases of our modern era.
CAR T-cell therapy for MS is an exciting frontier, with early data suggesting feasibility, safety, and potential efficacy through deep B-cell depletion in the CNS. While direct MS trial results are limited, insights from related autoimmune diseases like SLE and systemic sclerosis provide a strong foundation for optimism.
Two types of receptors are being researched. One approach would be to have CAR-T cells express a CD19 B- cell receptor. While we currently have highly effective B-cell therapies for MS, the hope is that the CAR-T cells would be even more effective by targeting inflammatory cells in the CNS directly.
Unlike cancer settings, autoimmune diseases require only transient CAR T-cell persistence to reset the immune system, reducing long-term risks like immune dysfunction or secondary malignancies. Short-term B-cell aplasia (weeks to months) leaves patients temporarily immunocompromised but allows healthy B-cell repopulation.
Cost and Accessibility: CAR T therapy is expensive and resource-intensive, requiring chemotherapy preconditioning and specialized manufacturing. However, a single infusion could be cost-effective compared to lifelong immunosuppressive therapies if remission is durable. Also challenges like long-term durability, patient selection, and cost must be addressed. Ongoing trials will be critical to determining whether CAR T can transform MS treatment, potentially offering a “reset” for the immune system and sustained remission.
Lets hope and pray that soon CAR- T therapies will prove to be an effective tool in the fight against MS.
HSCT (Hematopoietic Stem Cell Transplantation) for Multiple Sclerosis is now available at JCI-USA accredited World Class Hospital in India at most affordable cost.

HSCT attempts to “reboot” the faulty immune system, which is responsible for damaging the brain and spinal cord in MS. At present the total cost of HSCT at major centres like Mexico and Russia cost more than 100,000 US Dollars including travel and stay. At world class HSCT Hospital India HSCT costs about 30,000 US Dollars all inclusive. Please click this link to know more about world’s most affordable HSCT treatment Please click this link to know more about world’s most affordable HSCT treatment
Why you should choose HSCT Hospital India for HSCT ?
 HSCT Hospital India is one of the finest private hospitals in India and Accredited by JCIUSA. Most Affordable, 30,000 US $ HSCT package includes complete treatment cost for 30 days inhospital stay in a deluxe private room, Doctors Fee, Tests and Consultations, Medicines, Consumables, Neuro-Physiotherapy and also Food and Laundry for both the patient and the attendant, Airport Transfers etc. Large number of MS patients from Europe, America and Australia already treated successfully. Click here to know more
HSCT Hospital India is one of the finest private hospitals in India and Accredited by JCIUSA. Most Affordable, 30,000 US $ HSCT package includes complete treatment cost for 30 days inhospital stay in a deluxe private room, Doctors Fee, Tests and Consultations, Medicines, Consumables, Neuro-Physiotherapy and also Food and Laundry for both the patient and the attendant, Airport Transfers etc. Large number of MS patients from Europe, America and Australia already treated successfully. Click here to know more Complete 30 day HSCT done in hospital. Private deluxe rooms are very well served for patient and attendant’s comfort and equipped with HEPA Filter with Triple Level Air Filtration. No outside hospital stay avoids risk of infection, 24 x 7 nursing care and best medical attention. Advanced HSCT for MS protocol used does not require any further chemo/ treatment after leaving the hospital. Click here to get complete details
Complete 30 day HSCT done in hospital. Private deluxe rooms are very well served for patient and attendant’s comfort and equipped with HEPA Filter with Triple Level Air Filtration. No outside hospital stay avoids risk of infection, 24 x 7 nursing care and best medical attention. Advanced HSCT for MS protocol used does not require any further chemo/ treatment after leaving the hospital. Click here to get complete details International and Globally Renowned Accreditations – HSCT Hospital India is accredited by the Joint Commission International, USA, National Accreditation Board for Hospitals and Healthcare Providers (NABH), and National Accreditation Board for Laboratories (NABL) for processes and high-quality patient care.
International and Globally Renowned Accreditations – HSCT Hospital India is accredited by the Joint Commission International, USA, National Accreditation Board for Hospitals and Healthcare Providers (NABH), and National Accreditation Board for Laboratories (NABL) for processes and high-quality patient care. Large number of MS patients from Europe, America and Australia have already been treated successfully at HSCT Hospital India. Click here to watch patient testimonial videos
Large number of MS patients from Europe, America and Australia have already been treated successfully at HSCT Hospital India. Click here to watch patient testimonial videos




