Stem Cell Transplant Effective for Select MS Patients
 Greater Benefits for Relapsing-Remitting MS Found with HSCT Therapy
Greater Benefits for Relapsing-Remitting MS Found with HSCT Therapy
Lesion load, disability improved for relapsing patients with highly active MS.
Stem cell transplantation was more effective than disease-modifying therapy (DMT) for patients with highly active relapsing-remitting multiple sclerosis (MS), a randomized clinical trial showed.
Over a median follow-up of 2 years, only three of 55 patients treated with non-myeloablative autologous hematopoietic stem cell transplantation (HSCT) had disease progression, compared with 34 of 55 patients on medication, according to Richard Burt, MD, of the Northwestern University Feinberg School of Medicine in Chicago, and colleagues.
Mean disability level improved in the HCST group during the first year, but worsened in the disease-modifying therapy (DMT) group, they reported in JAMA.
“This is the first randomized trial in relapsing-remitting MS patients of hematopoietic stem cell transplantation versus best-available DMT,” Burt told MedPage Today. “You need to look at the group we treated — they had aggressively relapsing MS with moderate disability — and the results were much better than standard drug therapies.”
Two newer drugs were not included in the study, Burt noted: ocrelizumab (Ocrevus), which had not been approved during the study enrollment period, and alemtuzumab (Lemtrada), due to the autoimmune effects it has been associated with. “Because we had a built-in cross-over in the study, we didn’t know what effect alemtuzumab might have if these patients crossed over to transplant,” he said.
The trial included 110 patients with relapsing-remitting MS who had at least two relapses while receiving drug treatment in the prior year. These patients had an Expanded Disability Status Scale (EDSS) score of 2.0 to 6.0 (EDSS scores range from 0-10, with 10=worst neurologic disability) and were randomized at 4 U.S., European, and South American centers from 2005 to 2016. Mean age was 36 years and 66% were female.
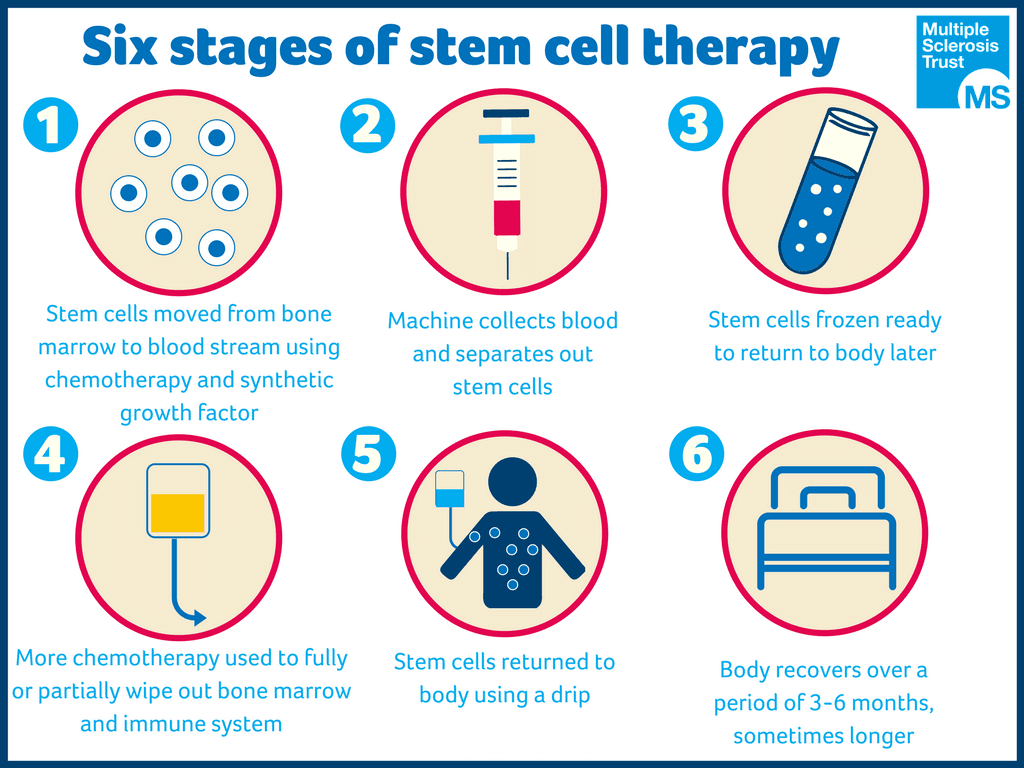
Patients in the stem cell transplantation group had a non-myeloablative regimen — a lower-dose, short course of more tolerable immune-specific chemotherapy and antibodies to suppress the immune system — and discontinued DMTs prior to the trial, with variable washout periods. Patients in the DMT group were treated with a mean of 1.3 different DMTs: 21 patients received natalizumab (Tysabri), 14 received dimethyl fumarate (Tecfidera), 14 received fingolimod (Gilenya), nine received glatiramer acetate (Copaxone), seven received interferon beta-1a (Rebif), six received mitoxantrone, and one received teriflunomide (Aubagio). Patients whose EDSS worsened with continued DMT treatment could cross over to the HSCT group.
The randomized clinical trial is the first to compare HSCT to DMTs.
During the first year, mean EDSS scores improved from 3.38 to 2.36 in the HSCT group and worsened from 3.31 to 3.98 in the DMT group (between-group mean difference -1.7, 95% CI -2.03 to -1.29, P<0.001).
Clinically significant disease progression also occurred much less frequently in the HSCT-treated group than the DMT-treated group (HR 0.07, 95% CI 0.02-0.24). Rates of disease progression at 1 year were 1.92% in the HSCT group and 24.5% in the DMT group. At 5 years, they were 9.71% in the HSCT group and 75.3% in the DMT group.
The HSCT group also had fewer relapses, improved MRI lesion load, a higher proportion of patients maintaining no evidence of disease activity, and a better quality of life. There were no deaths, and no patients who received HSCT developed non-hematopoietic grade 4 toxicities, such as myocardial infarction, sepsis, or other disabling or potential life-threatening events.
“Most comparative trials between DMTs measure benefit as a slowing of MS manifestations; this trial showed a global improvement over time in the mean level of disability and MRI lesion load in the HSCT-treated group while deterioration occurred in the DMT-treated group,” noted Harold Atkins, MD, of the University of Ottawa, in an accompanying editorial.
“Despite the positive results, several aspects of this trial deserve comment,” Atkins observed. While chemotherapy doses used in this study resulted in less severe toxicity than doses used for other malignant diseases, many patients experienced moderate to severe acute toxicity soon after HSCT, he stated. No treatment-related mortality was reported here or in other published cohorts of HSCT for MS performed in this manner, but “caution in patient selection remains warranted, as deaths have been reported using the same HSCT procedure in other autoimmune diseases,” he wrote.




